Schrödinger's Kitten
Irreverent Science for Everyone
How I fail to make Jelly
Every day, I try and make shape-changing jelly. Every day, my jelly does not work in new and different ways. I am not really sure why it is not working, and every failure opens more questions and variables. I am eating quite a lot of biscuits.
I hadn’t really internalised this aspect of a PhD. I was told they were hard, but I guess I thought that meant long hours. I’m not working long hours at the moment. I don't have enough ideas to fill them.
Why I want to make jelly
This is the issue. My project is to 3D print shape-changing materials. I looked at a lot of work on the subject (as of last May, already out of date) and decided I’d be working with heat-shrinking hydrogels (basically jelly) printed through an extrusion-based 3D printer. There’s a lot of material on hydrogels and how you can change how they respond1. The open source community has managed to mod printers to print marzipan, sugar, chocolate and clay, and kindly documented it all, so I had some hope I could make this work despite my lack of experience.
I’m by no stretch of the imagination of a chemist, so I picked a well-studied material, poly(N-isopropyl acrylamide) — henceforth PNIPAM — which is trivial to make into a gel, and changes shape quite dramatically, shrinking by about 50% when it passes 30 degrees or so. The fact it’s easy to make, biologically benign and easy to get to change quite dramatically accounts for its popularity. Also means I have loads of competition.
My plan is to make this material into a couple of different versions, that change shape at different rates. Then they can be squirted out of a 3D printer to make a multimaterial print which combines different gels. When I make a printed piece which changes size at different rates in different places, it will change shape: job’s a good’un.
How it is defying me
The problem is the material, really. I’m looking for something that’s ‘toothpaste-like’ — can be pushed out of a tube fairly easily, but just sits there boring and inert when not being pushed around, so I can stick other layers on top of it. Loads of things in the food industry do this. Think of fancy cake icing. Or watch some 3D printing videos. The technical term is a shear-thinning non Newtonian fluid, and, as mentioned, lots of hobbyist printers work with weird materials that fulfil these requirements. So, I thought, how hard can it be? Bitter laughter.
PNIPAM gels are made by mixing NIPAM monomers, a photoinitiator, and a crosslinker. When light of the right frequency hits the photoinitiator, it breaks up into free radicals. This makes the monomer inclined to form a bond with another monomer, releasing another radical, which then encourages another monomer to join the conga line, and so on. The outcome is a string of monomers — a polymer, which is joined into a 3D cargo net by the crosslinker, which connects strings of polymer together. The more connections in the net (net points, crosslinks), the stiffer the gel is and the less it can move, but the stronger it is. The fewer, the more fluid it is.
Attempt one: only crosslink PNIPAM a little bit, by only exposing it to a bit of UV. Did not work; PNIPAM is all or nothing, goes solid or stays liquid. A vial of it will be solid on one side and runny on the other with a sharp dividing line between the two. The solid doesn’t flow, and the runny bit is runny like water, not toothpaste: unsuitable for layering.
At this point Zhu et al. released 3D printed shoals of tiny hydrogel microfish that swim around powered by peroxide emissions from their tails, and contain modified proteins that fluoresce green when they encounter pollutants.
Gelous.
Attempt two: stir PNIPAM while exposing it to UV for various different time periods with different amounts of photoinitiator and cross-linker, and try and reduce the all or nothing effect that way. Regrettably, PNIPAM doesn’t cross-link at all, even after 6x the normal exposure, if you stir it during. No, I don’t know why.
Attempt three: Revelation! Going solid is fine, in fact desirable, once it has been printed. So, if I can just make the liquid stage thicker and more non-Newtonian, I could still work with this. The food industry uses a lot of thickeners which have non-Newtonian properties, so I popped to Sainsbury’s and bought some. This made a thick material that could be extruded (tick) before any crosslinking, and then with exposure to UV formed a solid-ish shape. About the consistency of cooked egg white. And it changed shape with heating! COOKING WITH GAS.
At this point Bakarich et al demonstrated a 3D printed shape changing material which was way tougher than mine, made from seaweed, and self-healed. They used it to print a self-regulating valve. Did I mention I love passivetech?
Ointment fly one: While trying to repair big samples of this mixture, they grew skins, like old custard. These skins contracted so they formed little landscapes and mountains, in some cases even rolling up to reveal fresh unskinned material and making big flaps. I don’t know why, I just tried to cut samples around those bits.
Ointment fly two: When trying to test a statistically relevant sample of the slabs of jelly I’d managed to cut, the samples kept rupturing. I was prepared for them to be weak, but weak enough that they got freaked out and broke irreparably while just deforming under their own force is a bit too weak. So...
Attempt four: Try and replicate the work of Bakarich et al, combining a strong alginate based network with a thermoresponsive NIPAM network. The alginate should give strength, and the PNIPAM the response. First batch: dissolved. Second batch, very tough, consistency of jelly babies, no thermo response. Third batch, tiny thermal expansion (like pretty much everything when it heats up).
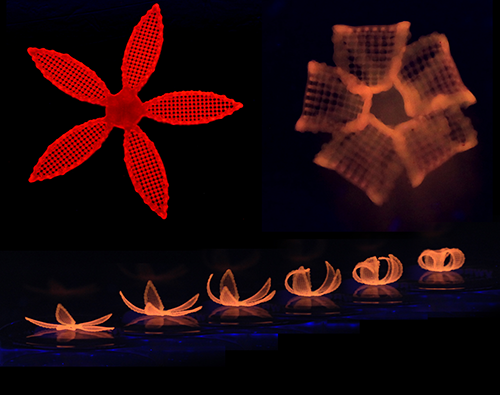
At this point Gladman et al released beautiful curvaceous glow in the dark flowers that reproduce any curved surface through tiny cellulose fibres aligned by the print direction. SIGH.
(An example of the curving flowers. Copyright Wyss Institute at Harvard.)
Wondered if the gels were now too tough and the heat response wasn’t strong enough to move them. Added less seaweed. Fourth batch, wrinkled surfaces and still liquid underneath. Fifth batch, just wrinkled, but coming apart when left to cure. No thermal expansion. Sixth batch, prepared on ice, wrinkled layer embedded in the middle of the jelly, no thermoresponse. Seventh batch, on ice, jelly hardly solid at all, no thermoresponse. Eighth batch, on ice, slight smooth white skin, shaped like cuttlefish for some reason (cast is cuboid, ???)
THERMORESPONSE!
Ninth batch, set off too early by the big UV reactor in the sky because the evenings are getting lighter. Tenth batch, did exactly the same, separated into wrinkly white skin and beige again. Eleventh batch, still separating, still wrinkly, now grainy too. Twelfth batch, wrinkly, not very solid, but same colour all the way through (I changed ratios each time, I wasn’t just doing the same thing and getting these weirdo results.) And it goes on...
The state of the art
Currently, my days consist of scowling at my experiments (what if I put in more cross linker and less alginate? moved the light closer? added ethanol? wore pink?), trying to describe the problems to other people and figure out what is going on (‘Is it normal for it to be off-white and wrinkly? My science, I mean.’), googling weird search terms (what is the science name for ‘custard skin’? And how does it happen?), scowling at my lab/office mates (‘How’s it going?’ ‘EVERYTHING IS AWFUL SEND METEORS’), and scowling at my review paper (don’t even ask).
You may be wondering why I am doing what I am doing, in the context of many talented people worldwide making things that frankly kick my ass. Well, firstly, I’m here to learn, and making a machine and a material are the skills I want to get, and if I am not the first or best or even notable in the world, I can still be proud of the thing I make and make good things in the future. Secondly, I might come up with a variation they haven’t done that might help someone else. Thirdly, most approaches to this use custom-built machines that cost a significant part of a million pounds. My system so far is under £700, made of off-the shelf parts, and my chemicals are cheap and easy. I use open source designs and will open source mine (once I get something working, open sourcing failures just seems like trolling). Fourthly, I took EPSRC’s shilling and feel I should do something for it.
So, if everyone would just stop printing lovely fish, flowers, and valves, and my gels would gel, and my thermoresponsive materials thermorespond, and my tubes didn’t explode in a poof of icing1, I might finally be able to get this sodding thing WORKING.
 RSS feed
RSS feed Twitter feed
Twitter feed